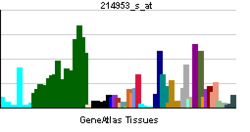
Amiloidni prekursorski protein


Amiloidni prekursorski protein (APP) je integralni membranski protein eksprimiran u mnogim tkivima i koncentriran u sinapsama neurona. Funkcioniše kao receptor ćelijske površine[5] i impliciran je kao regulator formiranja sinapsi,[6] plastičnost,[7] antimikrobu aktivnost,[8] i eksport gvožđa.[9] Kodiran je genom APP i reguliran prezentacijom supstrata.[10] APP je najpoznatiji kao prekursorska molekula čija proteoliza stvara amiloid beta (Aβ), polipeptid koji sadrži 37 do 49 aminokiselinskih ostataka, čiji amiloidni vlaknasti oblik je primarna komponenta amiloidnog plaka koji se nalazi u mozgu pacijenata s Alzheimerovom bolešću.
Genetika
[uredi | uredi izvor]Amiloid-beta prekursorski protein je drevna i visoko konzervirani protein.[11] U ljudi, gen APP nalazi se na hromosomu 21 i sadrži 18 egzona koji obuhvataju 290 kilobaza.[12][13] Kod ljudi primijećeno je nekoliko alternativno prerađenih izoformi APP-a, u rasponu dužine od 639 do 770 aminokiselina, s određenim izoformama prvenstveno eksprimiranim u neuronima; promjene u neuronskom omjeru ovih izoformi povezane su s Alzhheimerovom bolešću.[14] Homologni proteini identifikovani su u drugim organizmima kao što su u rodu Drosophila (vinske mušice), C. elegans (oble gliste),[15] i svi sisari.[16] Amiloidna beta regija proteina, koja se nalazi u domenu koji se prostire na membrani, nije dobro konzervirana među vrstama i nema očiglednu vezu sa biološkim funkcijama APP-a u nativnom stanju.[16]
Mutacije u kritičnim regijama amiloidnog prekursora proteina, uključujući regiju koja stvara amiloid-beta (Aβ), uzrokuju porodičnu osjetljivost na Alzheimerovu bolest.[17][18][19][20] Naprimjer, otkriveno je da nekoliko mutacija izvan Aβ regije povezanih s porodičnom Alzhejmerovom bolešću dramatično povećava proizvodnju Aβ.[21]
Mutacija A673T u APP genu štiti od Alzheimerove bolesti. Ova zamjena je u blizini mjesta cijepanja beta sekretaze i rezultira smanjenjem od 40% u stvaranju beta amiloida in vitro.[22]
Aminokiselinska sekvenca
[uredi | uredi izvor]Dužina polipeptidnog lanca je 770 aminokiselina, a molekulska težina 86.943 Da.[5]
| 10 | 20 | 30 | 40 | 50 | ||||
|---|---|---|---|---|---|---|---|---|
| MLPGLALLLL | AAWTARALEV | PTDGNAGLLA | EPQIAMFCGR | LNMHMNVQNG | ||||
| KWDSDPSGTK | TCIDTKEGIL | QYCQEVYPEL | QITNVVEANQ | PVTIQNWCKR | ||||
| GRKQCKTHPH | FVIPYRCLVG | EFVSDALLVP | DKCKFLHQER | MDVCETHLHW | ||||
| HTVAKETCSE | KSTNLHDYGM | LLPCGIDKFR | GVEFVCCPLA | EESDNVDSAD | ||||
| AEEDDSDVWW | GGADTDYADG | SEDKVVEVAE | EEEVAEVEEE | EADDDEDDED | ||||
| GDEVEEEAEE | PYEEATERTT | SIATTTTTTT | ESVEEVVREV | CSEQAETGPC | ||||
| RAMISRWYFD | VTEGKCAPFF | YGGCGGNRNN | FDTEEYCMAV | CGSAMSQSLL | ||||
| KTTQEPLARD | PVKLPTTAAS | TPDAVDKYLE | TPGDENEHAH | FQKAKERLEA | ||||
| KHRERMSQVM | REWEEAERQA | KNLPKADKKA | VIQHFQEKVE | SLEQEAANER | ||||
| QQLVETHMAR | VEAMLNDRRR | LALENYITAL | QAVPPRPRHV | FNMLKKYVRA | ||||
| EQKDRQHTLK | HFEHVRMVDP | KKAAQIRSQV | MTHLRVIYER | MNQSLSLLYN | ||||
| VPAVAEEIQD | EVDELLQKEQ | NYSDDVLANM | ISEPRISYGN | DALMPSLTET | ||||
| KTTVELLPVN | GEFSLDDLQP | WHSFGADSVP | ANTENEVEPV | DARPAADRGL | ||||
| TTRPGSGLTN | IKTEEISEVK | MDAEFRHDSG | YEVHHQKLVF | FAEDVGSNKG | ||||
| AIIGLMVGGV | VIATVIVITL | VMLKKKQYTS | IHHGVVEVDA | AVTPEERHLS | ||||
| KMQQNGYENP | TYKFFEQMQN |
Struktura
[uredi | uredi izvor]

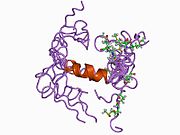
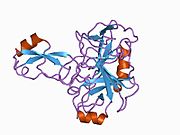
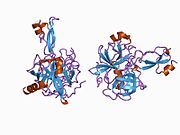
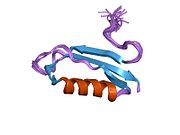
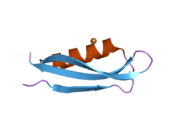
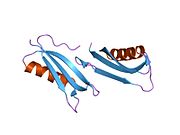
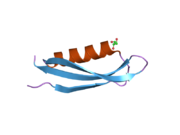
U APP sekvenci je identifikovan niz različitih, uglavnom nezavisno-sklopljenih strukturnih domena. Vanćelijska regija, mnogo veću od unutarćelijske regije, podijeljena je na E1 i E2 domene, povezane kiselim domenom (AcD); E1 sadrži dva poddomena uključujući domen sličan faktoru rasta (GFLD) i bakar-vezujući domen (CuBD) koji međusobno čvrsto djeluju.[24] Domen inhibitora serinske proteaze, odsutan iz izoforme različito eksprimirane u mozgu, nalazi se između kiselog područja i E2 domena.[25] Kompletna kristalna struktura APP-a još nije riješena. Međutim, pojedinačni domeni su uspješno kristalizirani poput: domen sličan faktoru rasta,[26], domena koja veže bakar[27] kompletna E1 domena[24] i domen E2.[23]
Funkcija
[uredi | uredi izvor]Iako je izvorna biološka uloga APP-a od očiglednog interesa za istraživanje Alzheimerove bolesti, temeljito razumijevanje je ostalo nedostižno.
Formiranje i popravak sinapsi
[uredi | uredi izvor]Najvažnija uloga APP-a je u m formiranju i popravljanju sinapsi;[6] njegova ekspresija je nadregulirana tokom neuronske diferencijacije i nakon nervne ozljede. Uloge u ćelijskoj signalizaciji, dugotrajnoj potenciaciji i ćelijskoj adheziji predložene su i podržane još ograničenim istraživanjem.[16] Konkretno, sličnosti u posttranslacijskoj obradi pozvali su na poređenja sa signalnom ulogom površinskog receptorskog proteina Notch.[28]
APP nokaut-miševi su održivi i imaju relativno male fenotipske efekte, uključujući oštećeno dugotrajno potenciranje i gubitak pamćenja bez općeg gubitka neurona.[29] S druge strane, zabilježeno je da transgeni miševi s povećanom ekspresijom APP-a pokazuju smanjenu dugotrajnu potencijaciju.[30]
Logičan zaključak je da bi, budući da se Aβ prekomjerno akumulira kod Alzheimerove bolesti, njegov prekursor, APP, također bio povišen. Međutim, tijela neuronskih ćelija sadrže manje APP kao funkciju njihove blizine amiloidnim plakovima.[31] Podaci pokazuju da je ovaj deficit u APP-u posljedica pada proizvodnje, a ne povećanja katalize. Gubitak APP neurona može uticati na fiziološke deficite koji doprinose demenciji.
Somatska rekombinacija
[uredi | uredi izvor]U neuronima ljudskog mozga, u genu koji kodira APP česta je somatska rekombinacija.[32] Neuroni osoba sa sporadičnom Alzheimmerovom bolešću pokazuju veću raznolikost gena APP zbog somatske rekombinacije nego neuroni zdravih osoba.[32]
Anterogradni neuronski transport
[uredi | uredi izvor]Molekule sintetizirane u ćelijskim tijelima neurona moraju se prenijeti prema van do distalnih sinapsi. Ovo se postiže putem brzog anterogradnog transporta. Utvrđeno je da APP može posredovati u interakciji između tereta i kinezina i na taj način olakšati ovaj transport. Konkretno, kratka peptidna sekvenca od 15 aminokiselina sa citoplazmatskog karboksi-terminala neophodna je za interakciju sa motornim proteinom.[33]
Dodatno, pokazalo se da je interakcija između APP i kinezina specifična za peptidnu sekvencu APP-a.[34] U nedavnom eksperimentu koji je uključivao transport obojenih kuglica, kontrole su konjugirane na jednu aminokiselinu, glicin, tako da pokazuju istu terminalnu grupu karboksilne kiseline kao APP bez intervencije gorepomenute 15-aminokiselinske sekvenca. Kontrolne kuglice nisu bile pokretne, što je pokazalo da terminalni COOH dio peptida nije dovoljan da posreduje u transportu.
Eksport gvožđa
[uredi | uredi izvor]Drugačiju perspektivu na Alzheimerovu bolest otkriva studija na mišu koja je otkrila da APP posjeduje feroksidaznu aktivnost sličnu ceruloplazminu, olakšavajući metabolizam i eksport gvožđa kroz interakciju sa feroportinom; čini se da je ova aktivnost blokirana cinkom zarobljenim akumuliranim Aβ kod Alzheimerove bolesti.[9] Pokazalo se da jednonukleotidni polimorfizam u 5' UTR-u APP iRNK može poremetiti njegovu translaciju.[35]
Hipoteza da APP ima aktivnost feroksidaze u domenu E2 i olakšava eksport Fe(II) je vjerovatno netačna jer predloženo mjesto feroksidaze APP u E2 domenu nema aktivnost feroksidaze.[36][37]
Kako APP ne posjeduje aktivnost feroksidaze unutar svog E2 domena, mehanizam APP-moduliranog efluksa gvožđa iz feroportina bio je pod kontrolom. Jedan model sugerira da APP djeluje na stabilizaciju proteina feroportina koji izlijeva gvožđe u ćelijskim plazmamembranama, čime se povećava ukupan broj feroportinskih molekula na membrani. Ovi transporteri gvožđa se zatim mogu aktivirati poznatim feroksidazama sisara (tj. ceruloplazminom ili hefestinom).[38]
Hormonska regulacija
[uredi | uredi izvor]Amiloid-β prekursorski protein (AβPP), i sve povezane sekretaze, eksprimiraju se u ranoj fazi razvoja i imaju ključnu ulogu u endokrinologiji reprodukcije – uz diferencijalnu obradu AβPP sekretazama koje regulišu proliferaciju ljudskih embrionskih matičnih ćelija (hESC) kao i njihovu diferencijaciju u nervne prekursorske ćelije (NPC). Hormon trudnoće ljudski horionski gonadotropin (hCG) povećava ekspresiju AβPP [39] i proliferaciju hESC-a, dok progesteron usmjerava preradu AβPP prema neamiloidogenom putu, koji promovira diferencijaciju hESC-a u NPC.[40][41][42]
AβPP i njegovi proizvodi cijepanja ne promovišu proliferaciju i diferencijaciju postmitotskih neurona; prije će biti da prekomjerna ekspresija bilo divljeg tipa ili mutantnog AβPP u postmitotskim neuronima inducira apoptozhnu smrt nakon njihovog ponovnog ulaska u ćelijski ciklus.[43] Pretpostavlja se da je gubitak spolnih steroida (uključujući progesteron), ali i povećanje razine luteinizirajućeg hormona, ekvivalent hCG-a za odrasle, nakon menopauze i tokom andropauze pokreće proizvodnju amiloida-β[44] i ponovni ulazak postmitotskih neurona u ćelijski ciklus.
Interakcije
[uredi | uredi izvor]Pokazalo se da protein prekursor amiloida reaguje sa:
APP stupa u interakciju i s reelinom, proteinom koji je uključen u brojne poremećaje mozga, uključujući Alzheimerovu bolest.[65]
Reference
[uredi | uredi izvor]- ^ a b c GRCh38: Ensembl release 89: ENSG00000142192 - Ensembl, maj 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000022892 - Ensembl, maj 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c "Amyloid-beta precursor protein". Pristupljeno 10. 1. 2021.
- ^ a b c Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J (Jul 2006). "Synapse formation and function is modulated by the amyloid precursor protein". The Journal of Neuroscience. 26 (27): 7212–21. doi:10.1523/JNEUROSCI.1450-06.2006. PMC 6673945. PMID 16822978.
- ^ Turner PR, O'Connor K, Tate WP, Abraham WC (maj 2003). "Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory". Progress in Neurobiology. 70 (1): 1–32. doi:10.1016/S0301-0082(03)00089-3. PMID 12927332. S2CID 25376584.
- ^ Moir RD, Lathe R, Tanzi RE (2018). "The antimicrobial protection hypothesis of Alzheimer's disease". Alzheimer's & Dementia. 14 (12): 1602–1614. doi:10.1016/j.jalz.2018.06.3040. PMID 30314800.
- ^ a b Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, Leong SL, Perez K, Johanssen T, Greenough MA, Cho HH, Galatis D, Moir RD, Masters CL, McLean C, Tanzi RE, Cappai R, Barnham KJ, Ciccotosto GD, Rogers JT, Bush AI (Sep 2010). "Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer's disease". Cell. 142 (6): 857–67. doi:10.1016/j.cell.2010.08.014. PMC 2943017. PMID 20817278.
- ^ Wang, Hao; Kulas, Joshua A.; Wang, Chao; Holtzman, David M.; Ferris, Heather A.; Hansen, Scott B. (17. 8. 2021). "Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol". Proceedings of the National Academy of Sciences. 118 (33): e2102191118. doi:10.1073/pnas.2102191118. PMC 8379952 Provjerite vrijednost parametra
|pmc=(pomoć). PMID 34385305 Provjerite vrijednost parametra|pmid=(pomoć). - ^ Tharp WG, Sarkar IN (april 2013). "Origins of amyloid-β". BMC Genomics. 14 (1): 290. doi:10.1186/1471-2164-14-290. PMC 3660159. PMID 23627794.
- ^ Yoshikai S, Sasaki H, Doh-ura K, Furuya H, Sakaki Y (Mar 1990). "Genomic organization of the human amyloid beta-protein precursor gene". Gene. 87 (2): 257–63. doi:10.1016/0378-1119(90)90310-N. PMID 2110105.
- ^ Lamb BT, Sisodia SS, Lawler AM, Slunt HH, Kitt CA, Kearns WG, Pearson PL, Price DL, Gearhart JD (Sep 1993). "Introduction and expression of the 400 kilobase amyloid precursor protein gene in transgenic mice [corrected]". Nature Genetics. 5 (1): 22–30. doi:10.1038/ng0993-22. PMID 8220418. S2CID 42752531.
- ^ Matsui T, Ingelsson M, Fukumoto H, Ramasamy K, Kowa H, Frosch MP, Irizarry MC, Hyman BT (Aug 2007). "Expression of APP pathway mRNAs and proteins in Alzheimer's disease". Brain Research. 1161: 116–23. doi:10.1016/j.brainres.2007.05.050. PMID 17586478. S2CID 26901380.
- ^ Ewald, Collin Y.; Li, Chris (1. 4. 2012). "Caenorhabditis elegans as a model organism to study APP function". Experimental Brain Research (jezik: engleski). 217 (3–4): 397–411. doi:10.1007/s00221-011-2905-7. ISSN 0014-4819. PMC 3746071. PMID 22038715.
- ^ a b c Zheng H, Koo EH (2006). "The amyloid precursor protein: beyond amyloid". Molecular Neurodegeneration. 1 (1): 5. doi:10.1186/1750-1326-1-5. PMC 1538601. PMID 16930452.
- ^ Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L (Feb 1991). "Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease". Nature. 349 (6311): 704–6. Bibcode:1991Natur.349..704G. doi:10.1038/349704a0. PMID 1671712. S2CID 4336069.
- ^ Murrell J, Farlow M, Ghetti B, Benson MD (Oct 1991). "A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease". Science. 254 (5028): 97–9. Bibcode:1991Sci...254...97M. doi:10.1126/science.1925564. PMID 1925564.
- ^ Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J (Oct 1991). "Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene". Nature. 353 (6347): 844–6. Bibcode:1991Natur.353..844C. doi:10.1038/353844a0. PMID 1944558. S2CID 4345311.
- ^ Lloyd, GM; Trejo-Lopez, JA; Xia, Y; McFarland, KN; Lincoln, SJ; Ertekin-Taner, N; Giasson, BI; Yachnis, AT; Prokop, S (12. 3. 2020). "Prominent amyloid plaque pathology and cerebral amyloid angiopathy in APP V717I (London) carrier - phenotypic variability in autosomal dominant Alzheimer's disease". Acta Neuropathologica Communications. 8 (1): 31. doi:10.1186/s40478-020-0891-3. PMC 7068954. PMID 32164763.
- ^ Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ (Dec 1992). "Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production". Nature. 360 (6405): 672–4. Bibcode:1992Natur.360..672C. doi:10.1038/360672a0. PMID 1465129. S2CID 4341170.
- ^ Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jönsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K (Aug 2012). "A mutation in APP protects against Alzheimer's disease and age-related cognitive decline". Nature. 488 (7409): 96–9. Bibcode:2012Natur.488...96J. doi:10.1038/nature11283. PMID 22801501. S2CID 4333449. Sažetak – The New York Times.
- ^ a b PDB 1RW6; Wang Y, Ha Y (Aug 2004). "The X-ray structure of an antiparallel dimer of the human amyloid precursor protein E2 domain". Molecular Cell. 15 (3): 343–53. doi:10.1016/j.molcel.2004.06.037. PMID 15304215.
- ^ a b Dahms SO, Hoefgen S, Roeser D, Schlott B, Gührs KH, Than ME (Mar 2010). "Structure and biochemical analysis of the heparin-induced E1 dimer of the amyloid precursor protein". Proceedings of the National Academy of Sciences of the United States of America. 107 (12): 5381–6. Bibcode:2010PNAS..107.5381D. doi:10.1073/pnas.0911326107. PMC 2851805. PMID 20212142.
- ^ Sisodia SS, Koo EH, Hoffman PN, Perry G, Price DL (Jul 1993). "Identification and transport of full-length amyloid precursor proteins in rat peripheral nervous system". The Journal of Neuroscience. 13 (7): 3136–42. doi:10.1523/JNEUROSCI.13-07-03136.1993. PMC 6576678. PMID 8331390.
- ^ Rossjohn J, Cappai R, Feil SC, Henry A, McKinstry WJ, Galatis D, Hesse L, Multhaup G, Beyreuther K, Masters CL, Parker MW (Apr 1999). "Crystal structure of the N-terminal, growth factor-like domain of Alzheimer amyloid precursor protein". Nature Structural Biology. 6 (4): 327–31. doi:10.1038/7562. PMID 10201399. S2CID 30925432.; see also PDB ID 1MWP
- ^ Kong GK, Adams JJ, Harris HH, Boas JF, Curtain CC, Galatis D, Masters CL, Barnham KJ, McKinstry WJ, Cappai R, Parker MW (Mar 2007). "Structural studies of the Alzheimer's amyloid precursor protein copper-binding domain reveal how it binds copper ions". Journal of Molecular Biology. 367 (1): 148–61. doi:10.1016/j.jmb.2006.12.041. PMID 17239395., 2FK2, 2FKL.
- ^ Selkoe D, Kopan R (2003). "Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration". Annual Review of Neuroscience. 26 (1): 565–97. doi:10.1146/annurev.neuro.26.041002.131334. PMID 12730322.
- ^ Phinney AL, Calhoun ME, Wolfer DP, Lipp HP, Zheng H, Jucker M (1999). "No hippocampal neuron or synaptic bouton loss in learning-impaired aged beta-amyloid precursor protein-null mice". Neuroscience. 90 (4): 1207–16. doi:10.1016/S0306-4522(98)00645-9. PMID 10338291. S2CID 6001957.
- ^ Matsuyama S, Teraoka R, Mori H, Tomiyama T (2007). "Inverse correlation between amyloid precursor protein and synaptic plasticity in transgenic mice". NeuroReport. 18 (10): 1083–7. doi:10.1097/WNR.0b013e3281e72b18. PMID 17558301. S2CID 34157306.
- ^ Barger SW, DeWall KM, Liu L, Mrak RE, Griffin WS (Aug 2008). "Relationships between expression of apolipoprotein E and beta-amyloid precursor protein are altered in proximity to Alzheimer beta-amyloid plaques: potential explanations from cell culture studies". Journal of Neuropathology and Experimental Neurology. 67 (8): 773–83. doi:10.1097/NEN.0b013e318180ec47. PMC 3334532. PMID 18648325.
- ^ a b Lee MH, Siddoway B, Kaeser GE, Segota I, Rivera R, Romanow WJ, Liu CS, Park C, Kennedy G, Long T, Chun J (novembar 2018). "Somatic APP gene recombination in Alzheimer's disease and normal neurons". Nature. 563 (7733): 639–645. Bibcode:2018Natur.563..639L. doi:10.1038/s41586-018-0718-6. PMC 6391999. PMID 30464338.
- ^ Satpute-Krishnan P, DeGiorgis JA, Conley MP, Jang M, Bearer EL (Oct 2006). "A peptide zipcode sufficient for anterograde transport within amyloid precursor protein". Proceedings of the National Academy of Sciences of the United States of America. 103 (44): 16532–7. Bibcode:2006PNAS..10316532S. doi:10.1073/pnas.0607527103. PMC 1621108. PMID 17062754.
- ^ Seamster PE, Loewenberg M, Pascal J, Chauviere A, Gonzales A, Cristini V, Bearer EL (Oct 2012). "Quantitative measurements and modeling of cargo-motor interactions during fast transport in the living axon". Physical Biology. 9 (5): 055005. Bibcode:2012PhBio...9e5005S. doi:10.1088/1478-3975/9/5/055005. PMC 3625656. PMID 23011729.
- ^ Rogers JT, Bush AI, Cho HH, Smith DH, Thomson AM, Friedlich AL, Lahiri DK, Leedman PJ, Huang X, Cahill CM (Dec 2008). "Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer's disease". Biochemical Society Transactions. 36 (Pt 6): 1282–7. doi:10.1042/BST0361282. PMC 2746665. PMID 19021541.
- ^ Ebrahimi KH, Hagedoorn PL, Hagen WR (2012). "A synthetic peptide with the putative iron binding motif of amyloid precursor protein (APP) does not catalytically oxidize iron". PLOS ONE. 7 (8): e40287. Bibcode:2012PLoSO...740287E. doi:10.1371/journal.pone.0040287. PMC 3419245. PMID 22916096.
- ^ Honarmand Ebrahimi K, Dienemann C, Hoefgen S, Than ME, Hagedoorn PL, Hagen WR (2013). "The amyloid precursor protein (APP) does not have a ferroxidase site in its E2 domain". PLOS ONE. 8 (8): e72177. Bibcode:2013PLoSO...872177H. doi:10.1371/journal.pone.0072177. PMC 3747053. PMID 23977245.
- ^ McCarthy RC, Park YH, Kosman DJ (Jul 2014). "sAPP modulates iron efflux from brain microvascular endothelial cells by stabilizing the ferrous iron exporter ferroportin". EMBO Reports. 15 (7): 809–15. doi:10.15252/embr.201338064. PMC 4196985. PMID 24867889.
- ^ Porayette P, Gallego MJ, Kaltcheva MM, Meethal SV, Atwood CS (Dec 2007). "Amyloid-beta precursor protein expression and modulation in human embryonic stem cells: a novel role for human chorionic gonadotropin". Biochemical and Biophysical Research Communications. 364 (3): 522–7. doi:10.1016/j.bbrc.2007.10.021. PMID 17959150.
- ^ Porayette P, Gallego MJ, Kaltcheva MM, Bowen RL, Vadakkadath Meethal S, Atwood CS (Aug 2009). "Differential processing of amyloid-beta precursor protein directs human embryonic stem cell proliferation and differentiation into neuronal precursor cells". The Journal of Biological Chemistry. 284 (35): 23806–17. doi:10.1074/jbc.M109.026328. PMC 2749153. PMID 19542221.
- ^ Gallego MJ, Porayette P, Kaltcheva MM, Meethal SV, Atwood CS (Jun 2009). "Opioid and progesterone signaling is obligatory for early human embryogenesis". Stem Cells and Development. 18 (5): 737–40. doi:10.1089/scd.2008.0190. PMC 2891507. PMID 18803462.
- ^ Gallego MJ, Porayette P, Kaltcheva MM, Bowen RL, Vadakkadath Meethal S, Atwood CS (2010). "The pregnancy hormones human chorionic gonadotropin and progesterone induce human embryonic stem cell proliferation and differentiation into neuroectodermal rosettes". Stem Cell Research & Therapy. 1 (4): 28. doi:10.1186/scrt28. PMC 2983441. PMID 20836886.
- ^ McPhie DL, Coopersmith R, Hines-Peralta A, Chen Y, Ivins KJ, Manly SP, Kozlowski MR, Neve KA, Neve RL (Jul 2003). "DNA synthesis and neuronal apoptosis caused by familial Alzheimer disease mutants of the amyloid precursor protein are mediated by the p21 activated kinase PAK3". The Journal of Neuroscience. 23 (17): 6914–27. doi:10.1523/JNEUROSCI.23-17-06914.2003. PMC 6740729. PMID 12890786.
- ^ Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS (maj 2004). "Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition". The Journal of Biological Chemistry. 279 (19): 20539–45. doi:10.1074/jbc.M311993200. PMID 14871891.
- ^ a b c Biederer T, Cao X, Südhof TC, Liu X (Sep 2002). "Regulation of APP-dependent transcription complexes by Mint/X11s: differential functions of Mint isoforms". The Journal of Neuroscience. 22 (17): 7340–51. doi:10.1523/JNEUROSCI.22-17-07340.2002. PMC 6757996. PMID 12196555.
- ^ a b Borg JP, Ooi J, Levy E, Margolis B (Nov 1996). "The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein". Molecular and Cellular Biology. 16 (11): 6229–41. doi:10.1128/mcb.16.11.6229. PMC 231626. PMID 8887653.
- ^ a b Araki Y, Tomita S, Yamaguchi H, Miyagi N, Sumioka A, Kirino Y, Suzuki T (Dec 2003). "Novel cadherin-related membrane proteins, Alcadeins, enhance the X11-like protein-mediated stabilization of amyloid beta-protein precursor metabolism". The Journal of Biological Chemistry. 278 (49): 49448–58. doi:10.1074/jbc.M306024200. PMID 12972431.
- ^ Tomita S, Ozaki T, Taru H, Oguchi S, Takeda S, Yagi Y, Sakiyama S, Kirino Y, Suzuki T (Jan 1999). "Interaction of a neuron-specific protein containing PDZ domains with Alzheimer's amyloid precursor protein". The Journal of Biological Chemistry. 274 (4): 2243–54. doi:10.1074/jbc.274.4.2243. PMID 9890987.
- ^ Tanahashi H, Tabira T (Feb 1999). "X11L2, a new member of the X11 protein family, interacts with Alzheimer's beta-amyloid precursor protein". Biochemical and Biophysical Research Communications. 255 (3): 663–7. doi:10.1006/bbrc.1999.0265. PMID 10049767.
- ^ Zambrano N, Buxbaum JD, Minopoli G, Fiore F, De Candia P, De Renzis S, Faraonio R, Sabo S, Cheetham J, Sudol M, Russo T (Mar 1997). "Interaction of the phosphotyrosine interaction/phosphotyrosine binding-related domains of Fe65 with wild-type and mutant Alzheimer's beta-amyloid precursor proteins". The Journal of Biological Chemistry. 272 (10): 6399–405. doi:10.1074/jbc.272.10.6399. PMID 9045663.
- ^ Guénette SY, Chen J, Jondro PD, Tanzi RE (Oct 1996). "Association of a novel human FE65-like protein with the cytoplasmic domain of the beta-amyloid precursor protein". Proceedings of the National Academy of Sciences of the United States of America. 93 (20): 10832–7. Bibcode:1996PNAS...9310832G. doi:10.1073/pnas.93.20.10832. PMC 38241. PMID 8855266.
- ^ Tanahashi H, Tabira T (Feb 1999). "Molecular cloning of human Fe65L2 and its interaction with the Alzheimer's beta-amyloid precursor protein". Neuroscience Letters. 261 (3): 143–6. doi:10.1016/S0304-3940(98)00995-1. PMID 10081969. S2CID 54307954.
- ^ Trommsdorff M, Borg JP, Margolis B, Herz J (Dec 1998). "Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein". The Journal of Biological Chemistry. 273 (50): 33556–60. doi:10.1074/jbc.273.50.33556. PMID 9837937.
- ^ Chow N, Korenberg JR, Chen XN, Neve RL (maj 1996). "APP-BP1, a novel protein that binds to the carboxyl-terminal region of the amyloid precursor protein". The Journal of Biological Chemistry. 271 (19): 11339–46. doi:10.1074/jbc.271.19.11339. PMID 8626687.
- ^ Zheng P, Eastman J, Vande Pol S, Pimplikar SW (Dec 1998). "PAT1, a microtubule-interacting protein, recognizes the basolateral sorting signal of amyloid precursor protein". Proceedings of the National Academy of Sciences of the United States of America. 95 (25): 14745–50. Bibcode:1998PNAS...9514745Z. doi:10.1073/pnas.95.25.14745. PMC 24520. PMID 9843960.
- ^ Wang B, Nguyen M, Breckenridge DG, Stojanovic M, Clemons PA, Kuppig S, Shore GC (Apr 2003). "Uncleaved BAP31 in association with A4 protein at the endoplasmic reticulum is an inhibitor of Fas-initiated release of cytochrome c from mitochondria". The Journal of Biological Chemistry. 278 (16): 14461–8. doi:10.1074/jbc.M209684200. PMID 12529377.
- ^ Lefterov IM, Koldamova RP, Lazo JS (Sep 2000). "Human bleomycin hydrolase regulates the secretion of amyloid precursor protein". FASEB Journal. 14 (12): 1837–47. doi:10.1096/fj.99-0938com. PMID 10973933. S2CID 44302063.
- ^ Araki Y, Miyagi N, Kato N, Yoshida T, Wada S, Nishimura M, Komano H, Yamamoto T, De Strooper B, Yamamoto K, Suzuki T (Jun 2004). "Coordinated metabolism of Alcadein and amyloid beta-protein precursor regulates FE65-dependent gene transactivation". The Journal of Biological Chemistry. 279 (23): 24343–54. doi:10.1074/jbc.M401925200. PMID 15037614.
- ^ Ikezu T, Trapp BD, Song KS, Schlegel A, Lisanti MP, Okamoto T (Apr 1998). "Caveolae, plasma membrane microdomains for alpha-secretase-mediated processing of the amyloid precursor protein". The Journal of Biological Chemistry. 273 (17): 10485–95. doi:10.1074/jbc.273.17.10485. PMID 9553108.
- ^ Hashimoto T, Wakabayashi T, Watanabe A, Kowa H, Hosoda R, Nakamura A, Kanazawa I, Arai T, Takio K, Mann DM, Iwatsubo T (Apr 2002). "CLAC: a novel Alzheimer amyloid plaque component derived from a transmembrane precursor, CLAC-P/collagen type XXV". The EMBO Journal. 21 (7): 1524–34. doi:10.1093/emboj/21.7.1524. PMC 125364. PMID 11927537.
- ^ Ohsawa I, Takamura C, Kohsaka S (Mar 2001). "Fibulin-1 binds the amino-terminal head of beta-amyloid precursor protein and modulates its physiological function". Journal of Neurochemistry. 76 (5): 1411–20. doi:10.1046/j.1471-4159.2001.00144.x. PMID 11238726. S2CID 83321033.
- ^ Chauhan VP, Ray I, Chauhan A, Wisniewski HM (maj 1999). "Binding of gelsolin, a secretory protein, to amyloid beta-protein". Biochemical and Biophysical Research Communications. 258 (2): 241–6. doi:10.1006/bbrc.1999.0623. PMID 10329371.
- ^ Yan SD, Fu J, Soto C, Chen X, Zhu H, Al-Mohanna F, Collison K, Zhu A, Stern E, Saido T, Tohyama M, Ogawa S, Roher A, Stern D (Oct 1997). "An intracellular protein that binds amyloid-beta peptide and mediates neurotoxicity in Alzheimer's disease". Nature. 389 (6652): 689–95. Bibcode:1997Natur.389..689D. doi:10.1038/39522. PMID 9338779. S2CID 4343238.
- ^ Tarr PE, Roncarati R, Pelicci G, Pelicci PG, D'Adamio L (maj 2002). "Tyrosine phosphorylation of the beta-amyloid precursor protein cytoplasmic tail promotes interaction with Shc". The Journal of Biological Chemistry. 277 (19): 16798–804. doi:10.1074/jbc.M110286200. PMID 11877420.
- ^ Hoe HS, Lee KJ, Carney RS, Lee J, Markova A, Lee JY, Howell BW, Hyman BT, Pak DT, Bu G, Rebeck GW (Jun 2009). "Interaction of reelin with amyloid precursor protein promotes neurite outgrowth". The Journal of Neuroscience. 29 (23): 7459–73. doi:10.1523/JNEUROSCI.4872-08.2009. PMC 2759694. PMID 19515914. Sažetak – Alzheimer Research Forum.
Dopunska literatura
[uredi | uredi izvor]- Beyreuther K, Pollwein P, Multhaup G, Mönning U, König G, Dyrks T, Schubert W, Masters CL (Sep 1993). "Regulation and expression of the Alzheimer's beta/A4 amyloid protein precursor in health, disease, and Down's syndrome". Annals of the New York Academy of Sciences. 695 (1 Transduction): 91–102. doi:10.1111/j.1749-6632.1993.tb23035.x. PMID 8239320. S2CID 22058428.
- Straub JE, Guevara J, Huo S, Lee JP (Jun 2002). "Long time dynamic simulations: exploring the folding pathways of an Alzheimer's amyloid Abeta-peptide". Accounts of Chemical Research. 35 (6): 473–81. doi:10.1021/ar010031e. PMID 12069633.
- Annaert W, De Strooper B (2003). "A cell biological perspective on Alzheimer's disease". Annual Review of Cell and Developmental Biology. 18 (1): 25–51. doi:10.1146/annurev.cellbio.18.020402.142302. PMID 12142279.
- Koo EH (Nov 2002). "The beta-amyloid precursor protein (APP) and Alzheimer's disease: does the tail wag the dog?". Traffic. 3 (11): 763–70. doi:10.1034/j.1600-0854.2002.31101.x. PMID 12383342. S2CID 40411806.
- Van Nostrand WE, Melchor JP, Romanov G, Zeigler K, Davis J (Nov 2002). "Pathogenic effects of cerebral amyloid angiopathy mutations in the amyloid beta-protein precursor". Annals of the New York Academy of Sciences. 977 (1): 258–65. Bibcode:2002NYASA.977..258N. doi:10.1111/j.1749-6632.2002.tb04824.x. PMID 12480759. S2CID 22567664.
- Ling Y, Morgan K, Kalsheker N (Nov 2003). "Amyloid precursor protein (APP) and the biology of proteolytic processing: relevance to Alzheimer's disease". The International Journal of Biochemistry & Cell Biology. 35 (11): 1505–35. doi:10.1016/S1357-2725(03)00133-X. PMID 12824062.
- Kerr ML, Small DH (Apr 2005). "Cytoplasmic domain of the beta-amyloid protein precursor of Alzheimer's disease: function, regulation of proteolysis, and implications for drug development". Journal of Neuroscience Research. 80 (2): 151–9. doi:10.1002/jnr.20408. PMID 15672415. S2CID 31985212.
- Maynard CJ, Bush AI, Masters CL, Cappai R, Li QX (Jun 2005). "Metals and amyloid-beta in Alzheimer's disease". International Journal of Experimental Pathology. 86 (3): 147–59. doi:10.1111/j.0959-9673.2005.00434.x. PMC 2517409. PMID 15910549.
- Tickler AK, Wade JD, Separovic F (Aug 2005). "The role of Abeta peptides in Alzheimer's disease". Protein and Peptide Letters. 12 (6): 513–9. doi:10.2174/0929866054395905. PMID 16101387.
- Reinhard C, Hébert SS, De Strooper B (Dec 2005). "The amyloid-beta precursor protein: integrating structure with biological function". The EMBO Journal. 24 (23): 3996–4006. doi:10.1038/sj.emboj.7600860. PMC 1356301. PMID 16252002.
- Watson D, Castaño E, Kokjohn TA, Kuo YM, Lyubchenko Y, Pinsky D, Connolly ES, Esh C, Luehrs DC, Stine WB, Rowse LM, Emmerling MR, Roher AE (Dec 2005). "Physicochemical characteristics of soluble oligomeric Abeta and their pathologic role in Alzheimer's disease". Neurological Research. 27 (8): 869–81. doi:10.1179/016164105X49436. PMID 16354549. S2CID 25687818.
- Calinisan V, Gravem D, Chen RP, Brittin S, Mohandas N, Lecomte MC, Gascard P (2006). "New insights into potential functions for the protein 4.1 superfamily of proteins in kidney epithelium". Frontiers in Bioscience. 11 (1): 1646–66. doi:10.2741/1911. PMID 16368544.
- Vetrivel KS, Thinakaran G (Jan 2006). "Amyloidogenic processing of beta-amyloid precursor protein in intracellular compartments". Neurology. 66 (2 Suppl 1): S69–73. doi:10.1212/01.wnl.0000192107.17175.39. PMID 16432149. S2CID 35965729.
- Gallo C, Orlassino R, Vineis C (Feb 2006). "[Recurrent intraparenchimal haemorrhages in a patient with cerebral amyloidotic angiopathy: description of one autopsy case]". Pathologica. 98 (1): 44–7. PMID 16789686.
- Coulson EJ (Aug 2006). "Does the p75 neurotrophin receptor mediate Abeta-induced toxicity in Alzheimer's disease?". Journal of Neurochemistry. 98 (3): 654–60. doi:10.1111/j.1471-4159.2006.03905.x. PMID 16893414. S2CID 20879380.
- Menéndez-González M, Pérez-Pinera P, Martínez-Rivera M, Calatayud MT, Blázquez Menes B (2006). "APP processing and the APP-KPI domain involvement in the amyloid cascade". Neuro-Degenerative Diseases. 2 (6): 277–83. doi:10.1159/000092315. PMID 16909010. S2CID 45002038.
- Neve RL, McPhie DL (Apr 2007). "Dysfunction of amyloid precursor protein signaling in neurons leads to DNA synthesis and apoptosis". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1772 (4): 430–7. doi:10.1016/j.bbadis.2006.10.008. PMC 1862818. PMID 17113271.
- Chen X, Stern D, Yan SD (Dec 2006). "Mitochondrial dysfunction and Alzheimer's disease". Current Alzheimer Research. 3 (5): 515–20. doi:10.2174/156720506779025215. PMID 17168650.
- Caltagarone J, Jing Z, Bowser R (Apr 2007). "Focal adhesions regulate Abeta signaling and cell death in Alzheimer's disease". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1772 (4): 438–45. doi:10.1016/j.bbadis.2006.11.007. PMC 1876750. PMID 17215111.
- Wolfe MS (Feb 2007). "When loss is gain: reduced presenilin proteolytic function leads to increased Abeta42/Abeta40. Talking Point on the role of presenilin mutations in Alzheimer disease". EMBO Reports. 8 (2): 136–40. doi:10.1038/sj.embor.7400896. PMC 1796780. PMID 17268504.
Vanjski linkovi
[uredi | uredi izvor]- GeneReviews/NCBI/NIH/UW entry on Early-Onset Familial Alzheimer Disease
- Amyloid Protein Precursor na US National Library of Medicine Medical Subject Headings (MeSH)
- Entrez Gene: APP amyloid beta (A4) precursor protein (peptidase nexin-II, Alzheimer disease)
- Lokacija ljudskog genoma APP i stranica sa detaljima o genu APP u UCSC Genome Browseru.