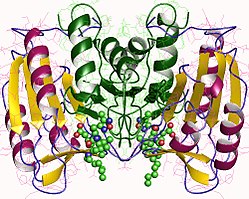
Kaspaza-3
Kaspaza-3 je kaspazni protein koji je u interakciji sa kaspazom-8 i kaspazom-9. Kodira ga gen "'CASP3"' koji se kod ljudi nalazi na hromosoma 4, sekvenca q35.1. CASP3 ortolozi[4] identificirani su kod brojnih sisara za koje su dostupni potpuni podaci o genomu. Jedinstveni ortolozi su također prisutni u ptica, guštera, Lissamphibia, i teleostea.
CASP3 protein je član porodice cistein-aspartata (kaspaza).[5] Sekvencna aktivacija kaspaza ima centralnu ulogu u fazi izvršenja ćelijske apoptoze. Kaspaze postoje kao neaktivni proenzimi koji se podvrgavaju proteolitskoj obradi na konzerviranim asparaginskim ostacima kako bi se proizvele dvije podjedinice, velika i mala, koje se dimeriziraju i formiraju aktivni enzim. Ovaj protein cijepa i aktivira kaspazu-6 i 7; a sam protein se obrađuje i aktivira kaspazama 8, 9 i 10. To je dominantna kaspaza uključena u cijepanje amiloid-beta 4A prekursorskog proteina, koja je povezana sa neuronskom smrću u Alzheimeromoj bolesti .[6] Alternativna prerada ovog gena rezultira u dvije varijante transkripta koje kodiraju isti protein.[7]
 |
 |
Kaspaza-3 dijeli mnoge tipske karakteristike zajedničke svim sada poznatim kaspazama. Naprimjer, njegovo aktivno mjesto sadrži cisteinski ostatak (Cys-163) i histidinski ostatak (His-121) koji stabiliziraju peptidnu vezu cijepanjem proteinske sekvence na karboksi-terminalnoj strani asparaginske kiseline kada je dio određene sekvence 4-amino kiseline.[9][10] Ova specifičnost omogućava kaspazama da budu nevjerovatno selektivne, sa 20.000 puta prednosti asparaginske kiseline u odnosu na glutaminsku.[11] Ključna karakteristika kaspaza u ćeliji je da su prisutne kao zimogeni, zvani prokaspaze, koje su neaktivne sve dok biohemijska promjena ne izazove njihovu aktivaciju. Svaka prokaspaza ima veliku N-terminalnu podjedinicu od oko 20 kDa, a zatim manju podjedinicu od oko 10 kDa, zvanu+e p20 i p10.[12]
Specifičnost supstrata[uredi | uredi izvor]
U normalnim okolnostima, kaspaze prepoznaju tetrapeptidne sekvence na svojim supstratima i hidroliziraju peptidne veze nakon ostataka asparaginske kiseline. Kaspaza 3 i kaspaza-7 dijele sličnu specifičnost supstrata, prepoznavanjem tetrapeptidnog motiva Asp-x-x-Asp.[13] C-terminal Asp je apsolutno neophodan dok se varijacije na ostala tri položaja mogu tolerisati.[14] Specifičnost supstrata kaspaze se široko koristi u kaspazi zasnovanoj na inhibitorima i dizajnu lijekova.[15]
Aminokiselinska sekvenca[uredi | uredi izvor]
Dužina polipeptidnog lanca je 277 aminokiselina, a molekulska težina 31.608 Da.[15]
| 10 | 20 | 30 | 40 | 50 | ||||
|---|---|---|---|---|---|---|---|---|
| MENTENSVDS | KSIKNLEPKI | IHGSESMDSG | ISLDNSYKMD | YPEMGLCIII | ||||
| NNKNFHKSTG | MTSRSGTDVD | AANLRETFRN | LKYEVRNKND | LTREEIVELM | ||||
| RDVSKEDHSK | RSSFVCVLLS | HGEEGIIFGT | NGPVDLKKIT | NFFRGDRCRS | ||||
| LTGKPKLFII | QACRGTELDC | GIETDSGVDD | DMACHKIPVE | ADFLYAYSTA | ||||
| PGYYSWRNSK | DGSWFIQSLC | AMLKQYADKL | EFMHILTRVN | RKVATEFESF | ||||
| SFDATFHAKK | QIPCIVSMLT | KELYFYH |
Struktura[uredi | uredi izvor]
Kaspaza-3, posebno, (također poznata kao CPP32/Yama/apopain)[16][17][18] formira se od zimogena od 32 kDa koji se cijepa na podjedinice od 17 kDa i 12 kDa. Kada se prokapaza cijepa na određenom ostatku, aktivni heterotetramer se tada može formirati hidrofobnim interakcijama, uzrokujući da se četiri antiparalelna beta-lista od p17 i dva od p12 spoje kako bi napravili heterodimer, koji zauzvrat stupa u interakciju s drugim heterodimerom da formira punu 12-lančanu beta-listnu strukturu okruženu alfa-heliksima koja je jedinstvena za kaspaze.[12][19] Kada se heterodimeri poravnaju jedan s drugim, aktivno mjesto je pozicionirano na svakom kraju molekule formirane od ostataka iz obje sudioničke podjedinice, iako se neophodni ostaci Cys-163 i His-121 nalaze na p17 (većoj) podjedinici.[19]

Mehanizam[uredi | uredi izvor]
Katalitsko mjesto kaspaze-3 uključuje tiolnu grupu Cys-163 i imidazolski prsten His-121. His-121 stabilizira karbonil grupu ključnog aspartatnog ostatka, dok Cys-163 napada kako bi konačno cijepao peptidnu vezu. Cys-163 i Gly-238 takođe funkcionišu da stabilizuju tetraedarsko prijelazno stanje kompleksa supstrat-enzim putem vodikove veze.[19] In vitro, utvrđeno je da kaspaza -3 preferira peptidnu sekvencu DEVDG (Asp-Glu-Val-Asp-Gly) sa cijepanjem koje se događa na karboksi strani drugog ostatka asparaginske kiseline (između D i G).[20]

Aktivacija[uredi | uredi izvor]
Kaspaza-3 se aktivira u apoptotskoj ćeliji i vanjskimim (ligand smrti) i unutrašnjim (mitohondrijskim) putevima.[12][21] The zymogen feature of caspase-3 is necessary because if unregulated, caspase activity would kill cells indiscriminately.[22] Kao egzekutorska kaspaza, zimogen kaspaze-3 nema praktično nikakvu aktivnost sve dok ga kaspaza inicijatora ne rascijepi nakon što su se dogodili događaji apoptotske signalizacije.[23] Jedan takav signalni događaj je uvođenje granzima B, koji može aktivirati inicijatorske kaspaze, u ćelije ciljane na apoptozu putem T-ćelija.[24][25] Ova vanjska aktivacija zatim pokreće osobenu kaspaznu kaskadu karakterističnu za apoptotski put, u kojoj kaspaza-3 ima dominantnu ulogu. U unutrašnjoj aktivaciji, citohrom c iz mitohondrija djeluje u kombinaciji sa kaspazom-9, faktorom 1 koji aktivira apoptozu (Apaf-1) i ATP za obradu prokapaze-3.[26] Ove molekule su dovoljne da aktiviraju kaspazu-3 in vitro, ali su neophodni i drugi regulatorni proteini in vivo.[26]
Pokazalo se da ekstrakt mangostina (Garcinia mangostana) inhibira aktivaciju kaspaze-3 u ljudskim neuronskim ćelijama tretiranim B-amiloidom.[27]
Inhibicija[uredi | uredi izvor]
Jedan od načina inhibicije kaspaze je preko porodice proteina IAP (inhibitor apoptoze), koja uključuje c-IAP1, c-IAP2, XIAP i ML-IAP.[19] XIAP binds and inhibits initiator caspase-9, which is directly involved in the activation of executioner caspase-3.[26] Međutim, tokom kaskade kaspaze, kaspaza-3 funkcioniše tako da inhibira aktivnost XIAP, cijepajući kaspazu-9 na određenom mjestu, sprječavajući XIAP da se veže i inhibira aktivnost kaspaze-9.[28]
Interakcije[uredi | uredi izvor]
Pokazalo se da je kaspaza-3 u interakciji sa:
Klinički značaj[uredi | uredi izvor]
Utvrđeno je da je kaspaza-3 neophodna za normalan razvoj mozga, kao i njenu tipsku ulogu u apoptozi, gdje je odgovorna za kondenzaciju hromatina i fragmentaciju DNK. Povišeni nivoi fragmenta kaspaze-3, p17, u krvotoku je znak nedavnog infarkta miokarda.[50] Sada je pokazano da kaspaza-3 može imati ulogu u embrionskoj i hematopoetskoj diferencijaciji matičnih ćelija.[51]
Također pogledajte[uredi | uredi izvor]
Reference[uredi | uredi izvor]
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000031628 - Ensembl, maj 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "OrthoMaM phylogenetic marker: CASP3 coding sequence". Arhivirano s originala, 3. 3. 2016. Pristupljeno 20. 12. 2009.
- ^ Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J (oktobar 1996). "Human ICE/CED-3 protease nomenclature". Cell. 87 (2): 171. doi:10.1016/S0092-8674(00)81334-3. PMID 8861900. S2CID 5345060.
- ^ Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW (april 1999). "Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid-beta precursor protein and amyloidogenic A beta peptide formation". Cell. 97 (3): 395–406. doi:10.1016/s0092-8674(00)80748-5. PMID 10319819. S2CID 17524567.
- ^ "Entrez Gene: CASP3 caspase 3, apoptosis-related cysteine peptidase".
- ^ Harrington HA, Ho KL, Ghosh S, Tung KC (2008). "Construction and analysis of a modular model of caspase activation in apoptosis". Theoretical Biology & Medical Modelling. 5 (1): 26. doi:10.1186/1742-4682-5-26. PMC 2672941. PMID 19077196.
- ^ Wyllie AH (1997). "Apoptosis: an overview". British Medical Bulletin. 53 (3): 451–65. doi:10.1093/oxfordjournals.bmb.a011623. PMID 9374030.
- ^ Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG, Hannun YA (juli 1997). "Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis". The Journal of Biological Chemistry. 272 (30): 18530–3. doi:10.1074/jbc.272.30.18530. PMID 9228015.
- ^ Stennicke HR, Renatus M, Meldal M, Salvesen GS (septembar 2000). "Internally quenched fluorescent peptide substrates disclose the subsite preferences of human caspases 1, 3, 6, 7 and 8". The Biochemical Journal. 350 (2): 563–8. doi:10.1042/0264-6021:3500563. PMC 1221285. PMID 10947972.
- ^ a b c Salvesen GS (januar 2002). "Caspases: opening the boxes and interpreting the arrows". Cell Death and Differentiation. 9 (1): 3–5. doi:10.1038/sj.cdd.4400963. PMID 11803369. S2CID 31274387.
- ^ Agniswamy J, Fang B, Weber IT (septembar 2007). "Plasticity of S2-S4 specificity pockets of executioner caspase-7 revealed by structural and kinetic analysis". The FEBS Journal. 274 (18): 4752–65. doi:10.1111/j.1742-4658.2007.05994.x. PMID 17697120. S2CID 1860924.
- ^ Fang B, Boross PI, Tozser J, Weber IT (juli 2006). "Structural and kinetic analysis of caspase-3 reveals role for s5 binding site in substrate recognition". Journal of Molecular Biology. 360 (3): 654–66. doi:10.1016/j.jmb.2006.05.041. PMID 16781734.
- ^ a b Weber IT, Fang B, Agniswamy J (oktobar 2008). "Caspases: structure-guided design of drugs to control cell death". Mini Reviews in Medicinal Chemistry. 8 (11): 1154–62. doi:10.2174/138955708785909899. PMID 18855730.
- ^ Fernandes-Alnemri T, Litwack G, Alnemri ES (decembar 1994). "CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme". The Journal of Biological Chemistry. 269 (49): 30761–4. doi:10.1016/S0021-9258(18)47344-9. PMID 7983002.
- ^ Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM (juni 1995). "Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase". Cell. 81 (5): 801–9. doi:10.1016/0092-8674(95)90541-3. PMID 7774019. S2CID 18866447.
- ^ Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA (juli 1995). "Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis". Nature. 376 (6535): 37–43. Bibcode:1995Natur.376...37N. doi:10.1038/376037a0. PMID 7596430. S2CID 4240789.
- ^ a b c d Lavrik IN, Golks A, Krammer PH (oktobar 2005). "Caspases: pharmacological manipulation of cell death". The Journal of Clinical Investigation. 115 (10): 2665–72. doi:10.1172/JCI26252. PMC 1236692. PMID 16200200.
- ^ Stennicke HR, Salvesen GS (oktobar 1997). "Biochemical characteristics of caspases-3, -6, -7, and -8". The Journal of Biological Chemistry. 272 (41): 25719–23. doi:10.1074/jbc.272.41.25719. PMID 9325297.
- ^ Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, Los M (august 2009). "Apoptosis and cancer: mutations within caspase genes". Journal of Medical Genetics. 46 (8): 497–510. doi:10.1136/jmg.2009.066944. PMID 19505876.
- ^ Boatright KM, Salvesen GS (decembar 2003). "Mechanisms of caspase activation". Current Opinion in Cell Biology. 15 (6): 725–31. doi:10.1016/j.ceb.2003.10.009. PMID 14644197.
- ^ Walters J, Pop C, Scott FL, Drag M, Swartz P, Mattos C, Salvesen GS, Clark AC (decembar 2009). "A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis". The Biochemical Journal. 424 (3): 335–45. doi:10.1042/BJ20090825. PMC 2805924. PMID 19788411.
- ^ Gallaher BW, Hille R, Raile K, Kiess W (septembar 2001). "Apoptosis: live or die--hard work either way!". Hormone and Metabolic Research. 33 (9): 511–9. doi:10.1055/s-2001-17213. PMID 11561209.
- ^ Katunuma N, Matsui A, Le QT, Utsumi K, Salvesen G, Ohashi A (2001). "Novel procaspase-3 activating cascade mediated by lysoapoptases and its biological significances in apoptosis". Advances in Enzyme Regulation. 41 (1): 237–50. doi:10.1016/S0065-2571(00)00018-2. PMID 11384748.
- ^ a b c Li P, Nijhawan D, Wang X (januar 2004). "Mitochondrial activation of apoptosis". Cell. 116 (2 Suppl): S57–9, 2 p following S59. doi:10.1016/S0092-8674(04)00031-5. PMID 15055583. S2CID 5180966.
- ^ Moongkarndi P, Srisawat C, Saetun P, Jantaravinid J, Peerapittayamongkol C, Soi-ampornkul R, Junnu S, Sinchaikul S, Chen ST, Charoensilp P, Thongboonkerd V, Neungton N (maj 2010). "Protective effect of mangosteen extract against beta-amyloid-induced cytotoxicity, oxidative stress and altered proteome in SK-N-SH cells" (PDF). Journal of Proteome Research. 9 (5): 2076–86. doi:10.1021/pr100049v. PMID 20232907.
- ^ Denault JB, Eckelman BP, Shin H, Pop C, Salvesen GS (juli 2007). "Caspase 3 attenuates XIAP (X-linked inhibitor of apoptosis protein)-mediated inhibition of caspase 9". The Biochemical Journal. 405 (1): 11–9. doi:10.1042/BJ20070288. PMC 1925235. PMID 17437405.
- ^ Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES (april 2002). "Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria". The Journal of Biological Chemistry. 277 (16): 13430–7. doi:10.1074/jbc.M108029200. PMID 11832478.
- ^ Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri ES (decembar 1996). "Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases". Proceedings of the National Academy of Sciences of the United States of America. 93 (25): 14486–91. Bibcode:1996PNAS...9314486S. doi:10.1073/pnas.93.25.14486. PMC 26159. PMID 8962078.
- ^ Selvakumar, P.; Sharma, RK. (maj 2007). "Role of calpain and caspase system in the regulation of N-myristoyltransferase in human colon cancer (Review)". Int J Mol Med. 19 (5): 823–7. doi:10.3892/ijmm.19.5.823. PMID 17390089.
- ^ Shu HB, Halpin DR, Goeddel DV (juni 1997). "Casper is a FADD- and caspase-related inducer of apoptosis". Immunity. 6 (6): 751–63. doi:10.1016/S1074-7613(00)80450-1. PMID 9208847.
- ^ Han DK, Chaudhary PM, Wright ME, Friedman C, Trask BJ, Riedel RT, Baskin DG, Schwartz SM, Hood L (oktobar 1997). "MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death". Proceedings of the National Academy of Sciences of the United States of America. 94 (21): 11333–8. Bibcode:1997PNAS...9411333H. doi:10.1073/pnas.94.21.11333. PMC 23459. PMID 9326610.
- ^ Forcet C, Ye X, Granger L, Corset V, Shin H, Bredesen DE, Mehlen P (mart 2001). "The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation". Proceedings of the National Academy of Sciences of the United States of America. 98 (6): 3416–21. Bibcode:2001PNAS...98.3416F. doi:10.1073/pnas.051378298. PMC 30668. PMID 11248093.
- ^ Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S (april 1999). "Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells". The EMBO Journal. 18 (8): 2040–8. doi:10.1093/emboj/18.8.2040. PMC 1171288. PMID 10205158.
- ^ Xanthoudakis S, Roy S, Rasper D, Hennessey T, Aubin Y, Cassady R, Tawa P, Ruel R, Rosen A, Nicholson DW (april 1999). "Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis". The EMBO Journal. 18 (8): 2049–56. doi:10.1093/emboj/18.8.2049. PMC 1171289. PMID 10205159.
- ^ Ruzzene M, Penzo D, Pinna LA (maj 2002). "Protein kinase CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) induces apoptosis and caspase-dependent degradation of haematopoietic lineage cell-specific protein 1 (HS1) in Jurkat cells". The Biochemical Journal. 364 (Pt 1): 41–7. doi:10.1042/bj3640041. PMC 1222543. PMID 11988074.
- ^ Chen YR, Kori R, John B, Tan TH (novembar 2001). "Caspase-mediated cleavage of actin-binding and SH3-domain-containing proteins cortactin, HS1, and HIP-55 during apoptosis". Biochemical and Biophysical Research Communications. 288 (4): 981–9. doi:10.1006/bbrc.2001.5862. PMID 11689006.
- ^ Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC (decembar 1998). "IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs". Cancer Research. 58 (23): 5315–20. PMID 9850056.
- ^ Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH (januar 2001). "An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7". Biochemistry. 40 (4): 1117–23. doi:10.1021/bi001603q. PMID 11170436.
- ^ Lee ZH, Lee SE, Kwack K, Yeo W, Lee TH, Bae SS, Suh PG, Kim HH (mart 2001). "Caspase-mediated cleavage of TRAF3 in FasL-stimulated Jurkat-T cells". Journal of Leukocyte Biology. 69 (3): 490–6. doi:10.1189/jlb.69.3.490. PMID 11261798. S2CID 34256107.
- ^ Leo E, Deveraux QL, Buchholtz C, Welsh K, Matsuzawa S, Stennicke HR, Salvesen GS, Reed JC (mart 2001). "TRAF1 is a substrate of caspases activated during tumor necrosis factor receptor-alpha-induced apoptosis". The Journal of Biological Chemistry. 276 (11): 8087–93. doi:10.1074/jbc.M009450200. PMID 11098060.
- ^ Suzuki Y, Nakabayashi Y, Takahashi R (juli 2001). "Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death". Proceedings of the National Academy of Sciences of the United States of America. 98 (15): 8662–7. Bibcode:2001PNAS...98.8662S. doi:10.1073/pnas.161506698. PMC 37492. PMID 11447297.
- ^ Silke J, Hawkins CJ, Ekert PG, Chew J, Day CL, Pakusch M, Verhagen AM, Vaux DL (april 2002). "The anti-apoptotic activity of XIAP is retained upon mutation of both the caspase 3- and caspase 9-interacting sites". The Journal of Cell Biology. 157 (1): 115–24. doi:10.1083/jcb.200108085. PMC 2173256. PMID 11927604.
- ^ Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, Liddington RC, Salvesen GS (mart 2001). "Structural basis for the inhibition of caspase-3 by XIAP". Cell. 104 (5): 791–800. doi:10.1016/S0092-8674(01)00274-4. PMID 11257232. S2CID 17915093.
- ^ Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC (decembar 1997). "The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases". The EMBO Journal. 16 (23): 6914–25. doi:10.1093/emboj/16.23.6914. PMC 1170295. PMID 9384571.
- ^ Deveraux QL, Takahashi R, Salvesen GS, Reed JC (juli 1997). "X-linked IAP is a direct inhibitor of cell-death proteases". Nature. 388 (6639): 300–4. Bibcode:1997Natur.388..300D. doi:10.1038/40901. PMID 9230442. S2CID 4395885.
- ^ Suzuki Y, Nakabayashi Y, Nakata K, Reed JC, Takahashi R (juli 2001). "X-linked inhibitor of apoptosis protein (XIAP) inhibits caspase-3 and -7 in distinct modes". The Journal of Biological Chemistry. 276 (29): 27058–63. doi:10.1074/jbc.M102415200. PMID 11359776.
- ^ Ohtsubo T, Kamada S, Mikami T, Murakami H, Tsujimoto Y (septembar 1999). "Identification of NRF2, a member of the NF-E2 family of transcription factors, as a substrate for caspase-3(-like) proteases". Cell Death and Differentiation. 6 (9): 865–72. doi:10.1038/sj.cdd.4400566. PMID 10510468.
- ^ Agosto M, Azrin M, Singh K, Jaffe AS, Liang BT (januar 2011). "Serum caspase-3 p17 fragment is elevated in patients with ST-segment elevation myocardial infarction: a novel observation". Journal of the American College of Cardiology. 57 (2): 220–1. doi:10.1016/j.jacc.2010.08.628. PMID 21211695.
- ^ Abdul-Ghani M, Megeney LA (juni 2008). "Rehabilitation of a contract killer: caspase-3 directs stem cell differentiation". Cell Stem Cell. 2 (6): 515–6. doi:10.1016/j.stem.2008.05.013. PMID 18522841.
Dopunska literatrura[uredi | uredi izvor]
- Cohen GM (august 1997). "Caspases: the executioners of apoptosis". The Biochemical Journal. 326 (Pt 1): 1–16. doi:10.1042/bj3260001. PMC 1218630. PMID 9337844.
- Roig J, Traugh JA (2001). Cytostatic p21 G protein-activated protein kinase gamma-PAK. Vitamins & Hormones. 62. str. 167–98. doi:10.1016/S0083-6729(01)62004-1. ISBN 9780127098623. PMID 11345898.
- Zhao LJ, Zhu H (decembar 2004). "Structure and function of HIV-1 auxiliary regulatory protein Vpr: novel clues to drug design". Current Drug Targets. Immune, Endocrine and Metabolic Disorders. 4 (4): 265–75. doi:10.2174/1568008043339668. PMID 15578977.
- Le Rouzic E, Benichou S (2006). "The Vpr protein from HIV-1: distinct roles along the viral life cycle". Retrovirology. 2 (1): 11. doi:10.1186/1742-4690-2-11. PMC 554975. PMID 15725353.
- Sykes MC, Mowbray AL, Jo H (februar 2007). "Reversible glutathiolation of caspase-3 by glutaredoxin as a novel redox signaling mechanism in tumor necrosis factor-alpha-induced cell death". Circulation Research. 100 (2): 152–4. doi:10.1161/01.RES.0000258171.08020.72. PMID 17272816.
Vanjski linkovi[uredi | uredi izvor]
| Commons ima datoteke na temu: Kaspaza-3 |
- The MEROPS online database for peptidases and their inhibitors: C14.003 Arhivirano 3. 3. 2016. na Wayback Machine
- Apoptosis & Caspase 3 – The Proteolysis Map-animation